Cancer early detection remains a critical challenge in modern healthcare, necessitating advanced predictive technologies to identify at-risk individuals before symptoms manifest. Artificial intelligence has emerged as a powerful solution to the complex puzzle of cancer risk assessment, yet implementation barriers persist due to inconsistent performance across diverse patient demographics and insufficient representation of minority populations in training datasets.
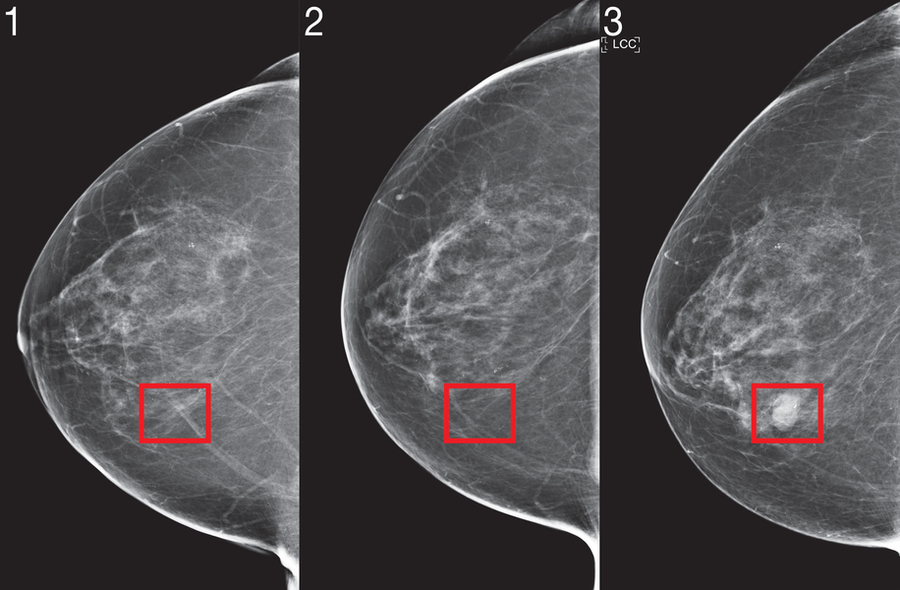
In a groundbreaking development two years ago, researchers from MIT's prestigious Computer Science and Artificial Intelligence Laboratory (CSAIL) in collaboration with Jameel Clinic unveiled an innovative deep learning framework capable of forecasting cancer susceptibility using exclusively mammogram imagery. This pioneering technology demonstrated remarkable potential while addressing healthcare disparities by maintaining equal accuracy for both Caucasian and African-American women—a particularly crucial advancement considering that Black women face a 43% higher mortality rate from breast cancer.
The transition from theoretical promise to clinical implementation demanded both sophisticated algorithmic enhancements and comprehensive multi-institutional validation to demonstrate the system's reliability across diverse healthcare environments. The research team recognized that for image-based risk assessment models to gain widespread clinical acceptance, they must undergo rigorous testing across multiple medical centers while maintaining consistent performance metrics.
To address these challenges, the scientists engineered their revolutionary "Mirai" algorithm specifically designed to meet the unique demands of contemporary risk modeling. This cutting-edge system simultaneously evaluates a patient's risk trajectory across multiple future time horizons while maintaining the flexibility to incorporate traditional clinical variables such as age or familial cancer history when available. Notably, Mirai's architecture ensures prediction consistency across minor variations in clinical settings, including different mammography equipment manufacturers and imaging protocols.
The research team trained Mirai using an extensive dataset comprising over 200,000 mammography examinations from Massachusetts General Hospital (MGH), subsequently validating its performance across diverse international test sets from Sweden's Karolinska Institute and Taiwan's Chang Gung Memorial Hospital. Following successful validation outcomes, Mirai has been implemented into clinical practice at MGH, with collaborative partners actively working to integrate this predictive technology into routine patient care protocols.
Comparative analyses revealed Mirai's superior performance over existing risk assessment methodologies across all three validation datasets. When evaluating high-risk patient cohorts within the MGH test population, the research team discovered that Mirai successfully identified nearly twice as many future cancer diagnoses compared to the current clinical standard, the Tyrer-Cuzick model. Furthermore, the algorithm demonstrated remarkable consistency across patients of different racial backgrounds, age demographics, and breast density classifications within the MGH dataset, while maintaining accuracy across various cancer subtypes in the Karolinska validation cohort.
Adam Yala, a CSAIL doctoral candidate and principal investigator of the research published in Science Translational Medicine, emphasizes that 'Enhanced breast cancer risk prediction models enable the development of precision screening approaches that facilitate earlier malignancy detection while minimizing unnecessary interventions and associated harms compared to conventional guidelines.' He further states, 'Our objective is to integrate these technological advancements into standard clinical practice. We're establishing collaborations with healthcare institutions across multiple continents—including Novant Health in North Carolina, Emory in Georgia, Maccabi in Israel, TecSalud in Mexico, Apollo in India, and Barretos in Brazil—to validate the model's performance across diverse populations and optimize clinical implementation strategies.'
Despite widespread implementation of breast cancer screening programs, significant controversy persists regarding optimal screening approaches. More intensive screening protocols aim to maximize early detection benefits, while reduced-frequency strategies prioritize minimizing false-positive results, psychological distress, and economic burdens for individuals who would never develop breast malignancies.
Contemporary clinical guidelines employ risk assessment models to determine appropriate candidates for supplementary imaging and magnetic resonance imaging (MRI) procedures. Various guidelines utilize different approaches—some relying solely on chronological age to determine screening frequency, while others incorporate multiple factors including hormonal profiles, genetic markers, and breast tissue density to guide further diagnostic interventions. Despite decades of research and refinement, risk prediction models currently implemented in clinical settings demonstrate only moderate accuracy levels.
Recent advances in deep learning-based mammography risk assessment have demonstrated promising potential for clinical transformation. To facilitate the translation of this technology into practical healthcare applications, the research team identified three critical innovations essential for next-generation risk modeling: integrated temporal risk assessment, optional incorporation of non-imaging clinical variables, and methodologies ensuring consistent performance across diverse clinical environments.
Risk prediction modeling inherently involves analyzing patients with varying follow-up durations and evaluating risk at multiple future time points—factors that determine screening frequency, supplementary imaging requirements, and potential preventive treatment considerations. While developing separate models for each time horizon represents one approach, this methodology frequently produces logically inconsistent risk projections, such as predicting a higher probability of cancer development within two years compared to five years. To overcome this fundamental limitation, the research team engineered their model to simultaneously predict risk across all time points using an innovative 'additive-hazard layer' architecture.
While primarily focused on mammogram analysis, the research team designed Mirai to optionally incorporate non-imaging risk variables such as age and hormonal factors when available—without requiring this information for model functionality. A conventional approach would involve integrating these factors as direct model inputs alongside imaging data, but this design would prevent utilization by the majority of healthcare facilities lacking this technological infrastructure, such as Karolinska and Chang Gung Memorial Hospital. To enable universal applicability while preserving the benefits of additional risk factors, Mirai predicts this information during training and utilizes its own predictive estimations when clinical data is unavailable. Since mammograms contain comprehensive health information, many traditional risk factors including age and menopausal status can be accurately predicted from imaging data alone. This architectural design enables global implementation across diverse healthcare settings, with the flexibility to incorporate additional information when available.
For deep-learning risk models to be integrated into clinical practice guidelines, they must demonstrate consistent performance across diverse healthcare environments, with predictions unaffected by minor variables such as specific mammography equipment manufacturers. Even within a single medical institution, the researchers discovered that conventional training methodologies failed to produce consistent predictions before and after mammography machine upgrades, as algorithms tended to learn environment-specific artifacts. To eliminate this bias, the team implemented an adversarial training scheme that specifically learns mammogram representations invariant to the clinical environment source, ensuring prediction consistency across diverse settings.
To validate these enhancements across diverse clinical settings, the scientists evaluated Mirai's performance using independent test sets from Sweden's Karolinska Institute and Taiwan's Chang Gung Memorial Hospital, demonstrating consistent predictive accuracy across international populations. The research team additionally conducted comprehensive performance analyses across racial demographics, age groups, and breast density categories within the MGH dataset, alongside evaluation across various cancer subtypes in the Karolinska data, revealing uniformly excellent performance across all patient subgroups.
Salewai Oseni, a breast surgical specialist at Massachusetts General Hospital uninvolved with the research, observes that 'African-American women continue to present with breast cancer at younger ages and frequently with more advanced disease progression.' She further notes that 'This phenomenon, combined with the higher prevalence of triple-negative breast cancer in this demographic, has resulted in disproportionately increased breast cancer mortality rates. This study demonstrates the development of a risk prediction model with notable accuracy across racial groups. The potential for clinical implementation is substantial.'
Mirai's operational methodology follows a sophisticated multi-stage process: Initially, the mammogram image undergoes processing through an advanced 'image encoder' that extracts meaningful features. Subsequently, each image representation, combined with information about its acquisition view, is integrated with other images from different perspectives to generate a comprehensive representation of the complete mammogram. Concurrently, the system predicts traditional risk factors using a Tyrer-Cuzick model framework (including variables such as age, body mass index, and hormonal factors)—employing predicted values when actual measurements are unavailable. Finally, utilizing this integrated information, the additive-hazard layer generates a patient's risk probability for each year over the subsequent five-year period.
While the current iteration of Mirai doesn't analyze patients' historical imaging results, the research team recognizes that temporal changes in imaging contain valuable predictive information. Future development directions include creating methodologies to effectively utilize patients' complete imaging histories. Similarly, the researchers note that model performance could be enhanced through incorporation of 'tomosynthesis,' an advanced X-ray technique for screening asymptomatic patients. Beyond accuracy improvements, additional investigation is required to adapt image-based risk models for different mammography devices when training data availability is limited.
'We understand that MRI technology can detect cancers earlier than mammography, and that earlier detection significantly improves patient outcomes,' explains Yala. 'However, for patients with low cancer risk profiles, the potential harms of false-positive findings may outweigh the benefits. With enhanced risk prediction models, we can develop more sophisticated screening guidelines that offer more sensitive detection methods like MRI to patients who will actually develop cancer, thereby improving outcomes while reducing unnecessary interventions and overtreatment for the remaining population.'
'We're both excited and humbled to investigate whether this AI system will effectively serve African-American populations,' states Judy Gichoya, MD, MS, and Assistant Professor of Interventional Radiology and Informatics at Emory University, who was not involved in the research. 'We're conducting extensive studies to address this critical question and develop methodologies for detecting potential failure points in diverse demographic applications.'
The collaborative research team behind Mirai includes Yala alongside MIT research specialist Peter G. Mikhael, radiologist Fredrik Strand of Karolinska University Hospital, Gigin Lin of Chang Gung Memorial Hospital, Associate Professor Kevin Smith of KTH Royal Institute of Technology, Professor Yung-Liang Wan of Chang Gung University, Leslie Lamb of MGH, Kevin Hughes of MGH, senior author and Harvard Medical School Professor Constance Lehman of MGH, and senior author and MIT Professor Regina Barzilay. This groundbreaking research received support from prestigious organizations including Susan G Komen, Breast Cancer Research Foundation, Quanta Computing, and the MIT Jameel Clinic, with additional funding provided by Chang Gung Medical Foundation Grant and Stockholm Läns Landsting HMT Grant.