In a groundbreaking development, researchers at the Massachusetts Institute of Technology (MIT) have engineered an innovative system that leverages artificial intelligence combined with advanced robotics to streamline the production of small molecules. This cutting-edge technology holds immense potential for applications across pharmaceutical development, renewable energy solutions, and advanced polymer materials.
Detailed in the August 8 publication of the prestigious journal Science, this revolutionary platform promises to liberate laboratory chemists from numerous repetitive and time-intensive procedures. Furthermore, it may unveil novel approaches to creating previously unexplored molecular compounds, according to project leaders Klavs F. Jensen, the Warren K. Lewis Professor of Chemical Engineering, and Timothy F. Jamison, the Robert R. Taylor Professor of Chemistry and associate provost at MIT.
"This innovation stands to transform how researchers approach molecular synthesis by eliminating the most tedious aspects of the process," Jensen explains. "This includes researching potential reaction pathways and constructing molecular assembly components for each new compound."
"Perhaps most excitingly, as a chemist, this system might inspire you to consider novel reactions you hadn't previously conceived," he adds.
The MIT research team featured in the Science publication includes Connor W. Coley, Dale A. Thomas III, Justin A. M. Lummiss, Jonathan N. Jaworski, Christopher P. Breen, Victor Schultz, Travis Hart, Joshua S. Fishman, Luke Rogers, Hanyu Gao, Robert W. Hicklin, Pieter P. Plehiers, Joshua Byington, John S. Piotti, William H. Green, and A. John Hart.
From Conceptualization to Creation: The Three-Stage Process
The novel system integrates three essential stages. Initially, AI-powered software proposes potential synthesis pathways for target molecules. Next, experienced chemists evaluate these routes and develop them into precise chemical "recipes." Finally, these recipes are transmitted to a robotic platform that autonomously assembles necessary equipment and executes the reactions required to construct the molecule.
Coley and his team have dedicated over three years to developing the open-source software suite that suggests and prioritizes potential synthesis routes. The software's foundation consists of multiple neural network models trained on millions of previously documented chemical reactions extracted from the Reaxys and U.S. Patent and Trademark Office databases. Leveraging this information, the software identifies reaction transformations and conditions it determines appropriate for constructing new compounds.
"The software assists in making high-level decisions regarding suitable intermediates and starting materials, followed by more detailed analyses of optimal reaction conditions and the likelihood of successful outcomes," Coley explains.
"A fundamental design principle of this software is its ability to extend beyond known molecules and reactions," he notes. "It can generalize to propose synthesis routes for entirely novel compounds that have never been synthesized before."
Following the software's synthesis suggestions, chemists refine these pathways to create comprehensive recipes for target molecules. This process sometimes requires laboratory experimentation or adjustments to reagent concentrations and reaction temperatures.
"Researchers draw inspiration from the AI's suggestions and transform them into executable recipe files, primarily because current chemical literature lacks sufficient detail to transition directly from concept to automated execution," Jamison explains.
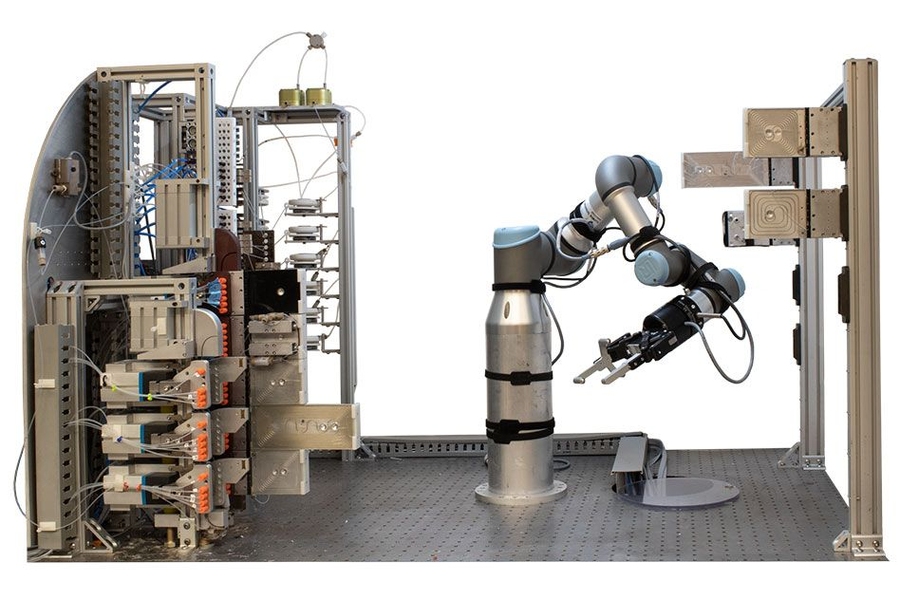
The finalized recipe is then loaded onto a platform where a robotic arm assembles modular reactors, separators, and other processing units into a continuous flow pathway, connecting pumps and lines that deliver molecular ingredients.
"You simply load the recipe—which controls the robotic platform—add the reagents, and initiate the process to generate your target molecule," Thomas describes. "Upon completion, the system automatically cleans itself, allowing you to load new reagents and recipes for subsequent runs."
Unlike the continuous flow system the researchers presented previously, which required manual reconfiguration after each synthesis, the new system is entirely configured by the robotic platform.
"This capability enables sequential production of multiple molecules and autonomous generation of molecular libraries," Jensen states.
The platform's design, approximately two cubic meters in size—slightly smaller than a standard chemical fume hood—resembles a telephone switchboard with an operator system that manages connections between platform modules.
"The robotic arm enables manipulation of fluidic pathways, which reduces the number of process modules and fluidic complexity," Thomas explains. "By decreasing fluidic complexity, we can increase molecular complexity, allowing for additional reaction steps within a relatively compact footprint."
Progressing Toward Complete Automation
The researchers evaluated the complete system by producing 15 different medicinal small molecules with varying synthesis complexity, with processes ranging from two hours for the simplest compounds to approximately 68 hours for manufacturing multiple molecules.
The team synthesized diverse compounds: aspirin and the antibiotic secnidazole in sequential processes; the painkiller lidocaine and the anti-anxiety medication diazepam in consecutive runs using shared reagents; the blood thinner warfarin and the Parkinson's disease medication safinamide, demonstrating the software's ability to design compounds with similar molecular components but different three-dimensional structures; and a family of five ACE inhibitor drugs and four nonsteroidal anti-inflammatory drugs.
"I'm particularly impressed by the diversity of chemistry and the variety of different chemical reactions the system can handle," Jamison notes, highlighting that the system managed approximately 30 different reactions compared to about 12 in their previous continuous flow system.
"We're actively working to bridge the gap between computational idea generation and practical synthesis execution," Coley explains. "We anticipate that next-generation systems will further enable scientists to focus their time and efforts on creativity and design."
The research received partial funding from the U.S. Defense Advanced Research Projects Agency (DARPA) Make-It program.